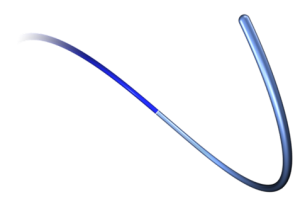
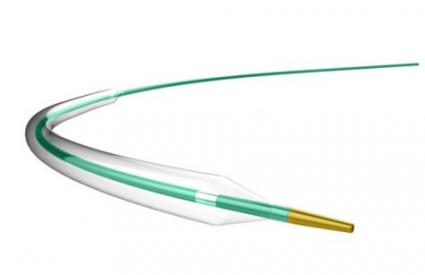
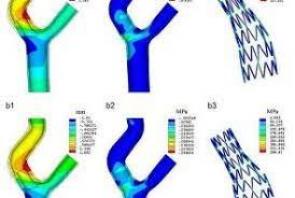
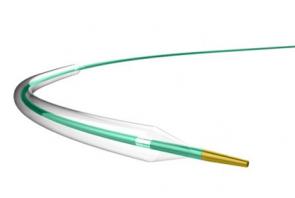
LAGUNA™ Aspiration Catheter
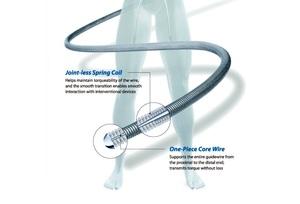
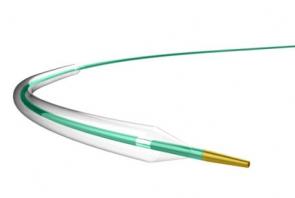
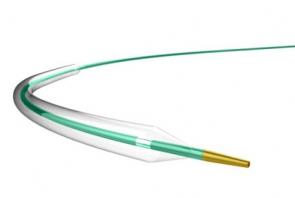
LAGUNA™ Aspiration Catheter is specifically designed for enhanced fast and accurate thrombus aspiration. Topas Advance offers an innovative elliptic cross-sectional distal form that optimizes the aspiration lumen and reduces friction with the guidewire catheter. Topas has one of the largest extraction lumen. Its smooth tip is specifically designed to increase deliverability and the innovative shaft brings excellent pushabilty. These features lead to enhanced efficacy with the highest aspiration speed, and excellent deliverability.